Sr (Strontium) is an element with position number 38 in the periodic table.
Located in the V period. Melting point: 769 ℃. Density: 2.63 g/cm3.
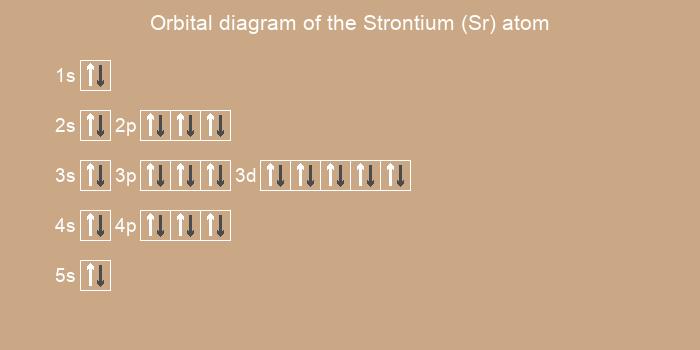
Electronic configuration of the Strontium atom in ascending order of orbital energies:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2
Electronic configuration of the Strontium atom in ascending order of the levels:
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2
Reduced electronic configuration Sr:
[Kr] 5s2
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2
Electronic configuration of the Strontium atom in ascending order of the levels:
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2
Reduced electronic configuration Sr:
[Kr] 5s2
Below is the electronic diagram of the Strontium atom
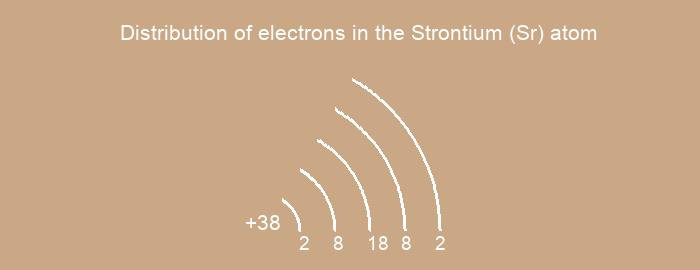
 Distribution of electrons over energy levels in the Sr atom
Distribution of electrons over energy levels in the Sr atom
1-st level (K): 2
2-st level (L): 8
3-st level (M): 18
4-st level (N): 8
5-st level (O): 2

1-st level (K): 2
2-st level (L): 8
3-st level (M): 18
4-st level (N): 8
5-st level (O): 2

Valence electrons of Strontium
The number of valence electrons in a Strontium atom - 2.
Below are their quantum numbers (N - energy, L - angular momentum, M - magnetic moment, S - spin )
Below are their quantum numbers (N - energy, L - angular momentum, M - magnetic moment, S - spin )
| Orbital | N | L | M | S |
|---|---|---|---|---|
| s | 5 | 0 | 0 | +1/2 |
| s | 5 | 0 | 0 | -1/2 |
Oxidation states of Strontium: +2
Other elements
Electronic configurations of other elements
Rubidium (Rb) electron configurationYttrium (Y) electron configuration
Electronic configuration table
