Pt (Platinum) is an element with position number 78 in the periodic table.
Located in the VI period. Melting point: 1772 ℃. Density: 21.45 g/cm3.
The order of filling the orbitals with electrons in the Pt atom is an exception to the rule.
Expected electronic configuration
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d8
But in reality, one electron moves from the 6s orbital to the 5d orbital:
Electronic configuration of the Platinum atom in ascending order of orbital energies:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1 4f14 5d9
Electronic configuration of the Platinum atom in ascending order of the levels:
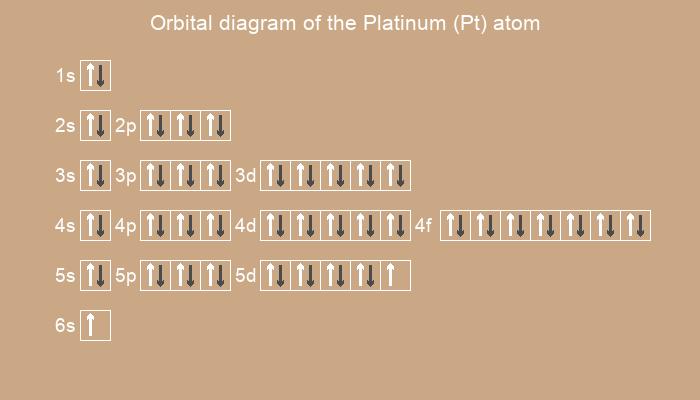
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d9 6s1
Reduced electronic configuration Pt:
[Xe] 4f14 5d9 6s1
Expected electronic configuration
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d8
But in reality, one electron moves from the 6s orbital to the 5d orbital:
Electronic configuration of the Platinum atom in ascending order of orbital energies:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1 4f14 5d9
Electronic configuration of the Platinum atom in ascending order of the levels:
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d9 6s1
Reduced electronic configuration Pt:
[Xe] 4f14 5d9 6s1
Below is the electronic diagram of the Platinum atom
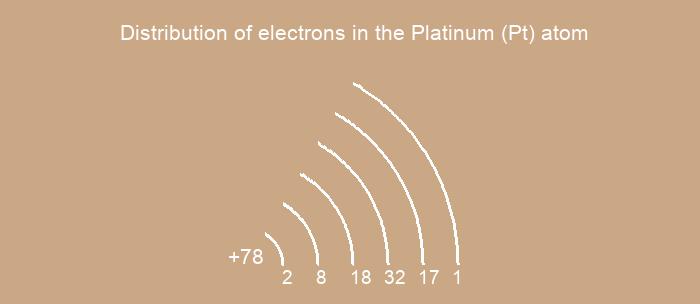
 Distribution of electrons over energy levels in the Pt atom
Distribution of electrons over energy levels in the Pt atom
1-st level (K): 2
2-st level (L): 8
3-st level (M): 18
4-st level (N): 32
5-st level (O): 17
6-st level (P): 1

1-st level (K): 2
2-st level (L): 8
3-st level (M): 18
4-st level (N): 32
5-st level (O): 17
6-st level (P): 1

Valence electrons of Platinum
The number of valence electrons in a Platinum atom - 10.
Below are their quantum numbers (N - energy, L - angular momentum, M - magnetic moment, S - spin )
Below are their quantum numbers (N - energy, L - angular momentum, M - magnetic moment, S - spin )
| Orbital | N | L | M | S |
|---|---|---|---|---|
| s | 6 | 0 | 0 | +1/2 |
| d | 5 | 2 | -2 | +1/2 |
| d | 5 | 2 | -1 | +1/2 |
| d | 5 | 2 | 0 | +1/2 |
| d | 5 | 2 | 1 | +1/2 |
| d | 5 | 2 | 2 | +1/2 |
| d | 5 | 2 | -2 | -1/2 |
| d | 5 | 2 | -1 | -1/2 |
| d | 5 | 2 | 0 | -1/2 |
| d | 5 | 2 | 1 | -1/2 |
Oxidation states of Platinum: +2, +4, +5, +6
Other elements
Electronic configurations of other elements
Iridium (Ir) electron configurationGold (Au) electron configuration
Electronic configuration table
