I (Iodine) is an element with position number 53 in the periodic table.
Located in the V period. Melting point: 113.5 ℃. Density: 4.94 g/cm3.
Electronic configuration of the Iodine atom in ascending order of orbital energies:
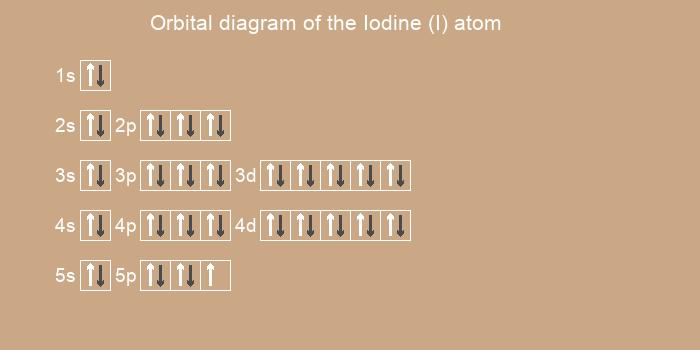
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
Electronic configuration of the Iodine atom in ascending order of the levels:
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5
Reduced electronic configuration I:
[Kr] 4d10 5s2 5p5
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
Electronic configuration of the Iodine atom in ascending order of the levels:
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5
Reduced electronic configuration I:
[Kr] 4d10 5s2 5p5
Below is the electronic diagram of the Iodine atom
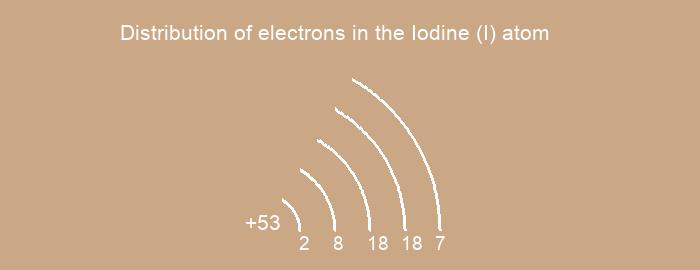
 Distribution of electrons over energy levels in the I atom
Distribution of electrons over energy levels in the I atom
1-st level (K): 2
2-st level (L): 8
3-st level (M): 18
4-st level (N): 18
5-st level (O): 7

1-st level (K): 2
2-st level (L): 8
3-st level (M): 18
4-st level (N): 18
5-st level (O): 7

Valence electrons of Iodine
The number of valence electrons in a Iodine atom - 7.
Below are their quantum numbers (N - energy, L - angular momentum, M - magnetic moment, S - spin )
Below are their quantum numbers (N - energy, L - angular momentum, M - magnetic moment, S - spin )
| Orbital | N | L | M | S |
|---|---|---|---|---|
| s | 5 | 0 | 0 | +1/2 |
| s | 5 | 0 | 0 | -1/2 |
| p | 5 | 1 | -1 | +1/2 |
| p | 5 | 1 | 0 | +1/2 |
| p | 5 | 1 | 1 | +1/2 |
| p | 5 | 1 | -1 | -1/2 |
| p | 5 | 1 | 0 | -1/2 |
Oxidation states of Iodine: -1, +1, +3, +5, +7
Other elements
Electronic configurations of other elements
Tellurium (Te) electron configurationXenon (Xe) electron configuration
Electronic configuration table
