Fe (Iron) is an element with position number 26 in the periodic table.
Located in the IV period. Melting point: 1535 ℃. Density: 7.87 g/cm3.
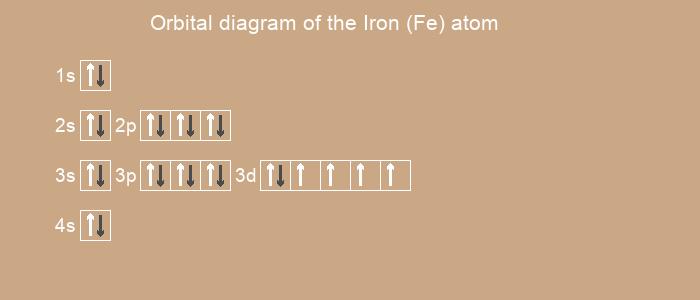
Electronic configuration of the Iron atom in ascending order of orbital energies:
1s2 2s2 2p6 3s2 3p6 4s2 3d6
Electronic configuration of the Iron atom in ascending order of the levels:
1s2 2s2 2p6 3s2 3p6 3d6 4s2
Reduced electronic configuration Fe:
[Ar] 3d6 4s2
1s2 2s2 2p6 3s2 3p6 4s2 3d6
Electronic configuration of the Iron atom in ascending order of the levels:
1s2 2s2 2p6 3s2 3p6 3d6 4s2
Reduced electronic configuration Fe:
[Ar] 3d6 4s2
Below is the electronic diagram of the Iron atom
 Distribution of electrons over energy levels in the Fe atom
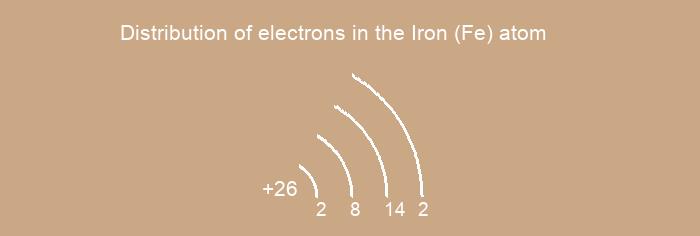
Distribution of electrons over energy levels in the Fe atom
1-st level (K): 2
2-st level (L): 8
3-st level (M): 14
4-st level (N): 2

1-st level (K): 2
2-st level (L): 8
3-st level (M): 14
4-st level (N): 2

Valence electrons of Iron
The number of valence electrons in a Iron atom - 8.
Below are their quantum numbers (N - energy, L - angular momentum, M - magnetic moment, S - spin )
Below are their quantum numbers (N - energy, L - angular momentum, M - magnetic moment, S - spin )
| Orbital | N | L | M | S |
|---|---|---|---|---|
| s | 4 | 0 | 0 | +1/2 |
| s | 4 | 0 | 0 | -1/2 |
| d | 3 | 2 | -2 | +1/2 |
| d | 3 | 2 | -1 | +1/2 |
| d | 3 | 2 | 0 | +1/2 |
| d | 3 | 2 | 1 | +1/2 |
| d | 3 | 2 | 2 | +1/2 |
| d | 3 | 2 | -2 | -1/2 |
Oxidation states of Iron: +1, +2, +3, +4, +5, +6
Other elements
Electronic configurations of other elements
Manganese (Mn) electron configurationCobalt (Co) electron configuration
Electronic configuration table
